SYSTEMIC SCLEROSIS : A-DUSE – Recruting
SPONSOR: CHU de Toulouse
COORDINATING/ PRINCIPAL INVESTIGATOR:
Pr. Grégory PUGNET
Service de Médecine Interne et immunologie clinique,
Hôpital Rangueil, 1 avenue J.Poulhès,
31059 Toulouse Cedex 9
Tel : 05 61 32 20 77
e-mail : pugnet.g@chu-toulouse.fr
Module Biothérapies CIC 1436 Toulouse :
Hôpital Rangueil, 1 Avenue Jean Poulhès,
31059 Toulouse Cedex 9
Marine LEBRIN-SERRENTINO ; Laetitia BUGAREL ; Fabian GROSS
Tel : 05 61 32 37 24
e-mails : lebrin.m@chu-toulouse.fr ; bugarel.l@chu-toulouse.fr; gross.f@chu-toulouse.fr
TITLE: Subcutaneous injections of cultured adipose-derived stroma/stem cells to heal refractory ischemic digital ulcers in patients with scleroderma: A phase II study (ADUSE)
CLINICAL TRIALS: NCT04356755
JUSTIFICATION/ CONTEXT
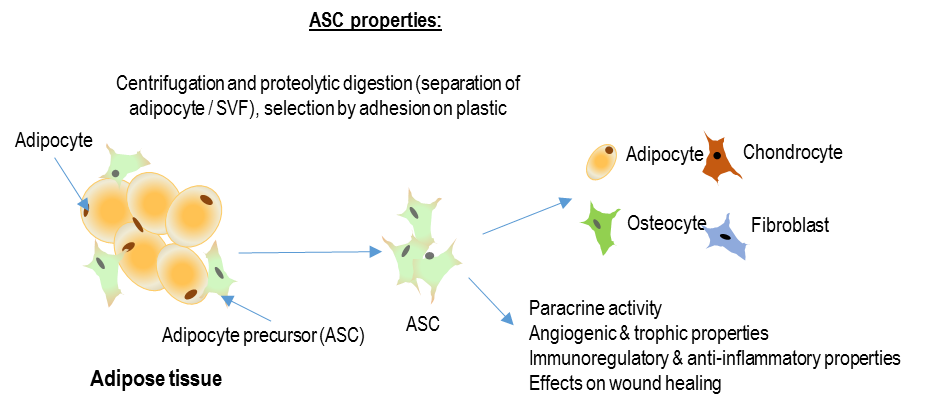
The hypothesis of our study is that digital injection of allogeneic cultured adipose-derived stromal cell (CellReady®) could be efficacious for scleroderma-induced refractory ischemic digital ulcers (DUs) healing by digital vascular regeneration and ulcer prevention in a clinical situation where no alternative therapy is validated
OBJECTIVES
Primary Objective:
Primary Objective:
To compare efficacy and safety of digital injection of allogeneic adipose-derived stromal cell (CellReady®) versus placebo for healing refractory (chronic and/or recurrent in the 3 months following a DU occurrence) active ischemic digital ulcers in patients with systemic sclerosis in a 16 weeks placebo- controlled trial.
Secondary objectives:
- To evaluate the efficacy of local injections of autologous cultured ASCs on the healing of DUs, the absence of complications or the appearance of new DUs, and on the quality of life and the pain scale of patients;
- To evaluate phenotype, cytokinin profile, immune-modulating and angiogenic activity of the injected adipose-derived stromal cells;
- Vascular biomarkers monitoring ;
- Bio-bank of serum and plasma.
CLINICAL TRIAL PROGRAM
INCLUSION CRITERIA
- Over 18 years old systemic sclerosis according to the 2013 ACR/EULAR classification criteria;
- At least one active digital ischemic ulcer located beyond the proximal interphalangeal joint, on finger surface (but not over subcutaneous calcifications or bone relief) and refractory after 10±2 weeks of standard of care;
- Women of childbearing potential must use a reliable method of contraception;
- Patient must have provided written informed consent prior to enrolment;
- Patient must be able to understand their requirements of participating in the protocol;
- Patient affiliated to a social security system.
EXCLUSION CRITERIA (non-exhaustive)
- Patients on statins, vasodilators, calcium channel blockers, ACE-inhibitors, nitroglycerin, α-adrenergic blockers, or angiotensin II receptor antagonists, N-acetylcysteine, antiplatelet aggregation therapy or low molecular weight heparin who have received treatment if present for less than 3 months or not stable for at least 1 month;
- Treatment with disease modifying agents such as methotrexate, mycophenolate, mofetil, azathioprine, tacrolimus, Interferons and cyclophosphamide;
- Treatment with oral corticosteroids (> 10 mg/day of prednisone or equivalent);
- Systemic antibiotics (oral and IV) to treat infected DU(s) within 4 weeks;
- Use of topical growth factors, hyperbaric oxygen;
- Local injection of botulinum toxin in an affected finger within 4 weeks prior to “inclusion visit”;
- Surgical sympathectomy of the upper limbs or surgical wound debridement within 1 month;
- Patient who underwent autologous hematopoietic stem cell transplantation (HSCT) within 1year;
- Patients with an indication for intensification by autologous HSCT;
- History of cancer in the last five years;
- Females who are pregnant or brestfeeding or plan to do so during the course of this study.
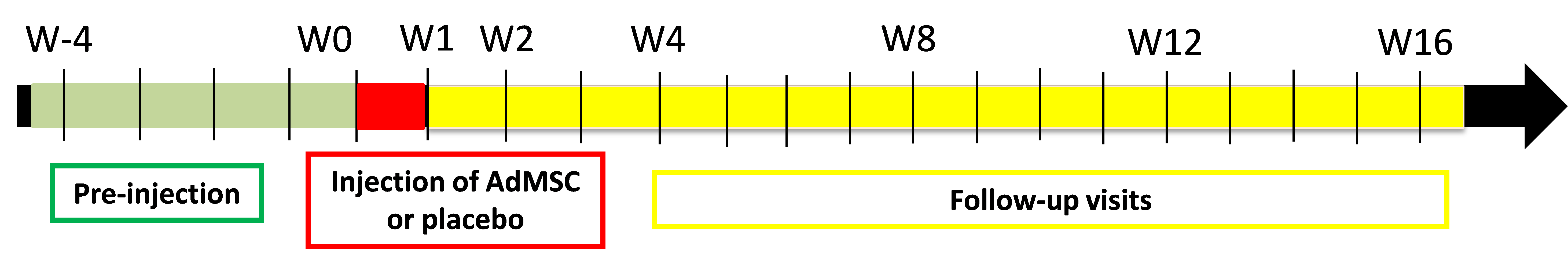
OUTLINE OF THE RESEARCH: Phase II, double-blind, randomized (1:1), multicenter, prospective, longitudinal, comparative, placebo-controlled clinical trial. Patients will be followed-up for 16 weeks.